
Cardinal Health Dragon Mask Aerosol Masks
$3.68
Dragon Mask Aerosol Masks are playful, soft and high-quality disposable pediatric masks which are made from high-grade resins....
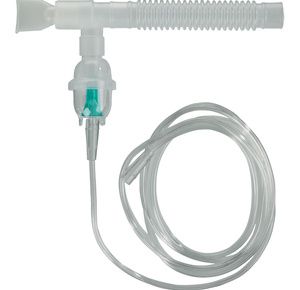
Drive Disposable Nebulizer Kit
$1.99
Drive Disposable Nebulizer Kit provides 80 percent of particles in respirable range....
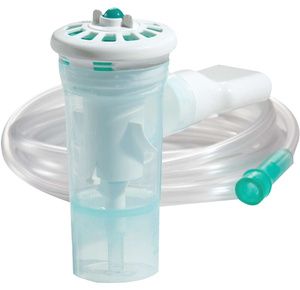
Monaghan AeroEclipse Reusable Breath Actuated Nebulizer (RBAN)
$24.99
AeroEclipse Reusable Breath Actuated Nebulizer (BAN) converts liquid medication into a fine mist so that it can be directly inhaled into the lungs....

Pari LC Plus Reusable Nebulizer
$17.79
Pari LC Plus uses Breath Enhanced Technology that delivers more medication when user breathes in and wastes less when user breathes out for the best treatment possible....
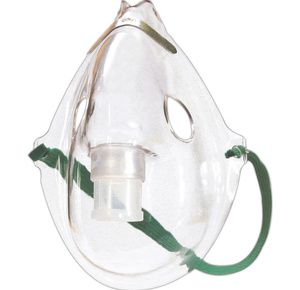
Drive Aerosol Mask
$1.04
It is a transparent mask made of soft plastic materials with an anatomical form. The mask maintains proper positioning behind the head to provide complete comfort....
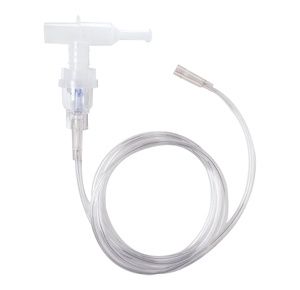
Medline Nebulizer Mouthpieces With Tubing
$2.42
Medline Nebulizer Mouthpieces comes with seven feet tube and is latex free....
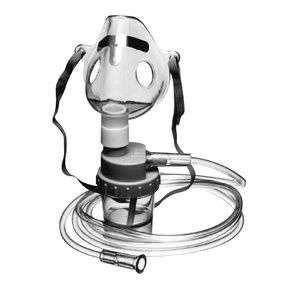
Allied Adult Mask With Nebulizer And 7 Feet Tubing
$4.99
Allied Adult Mask With Nebulizer And Tubing is designed for people with respiratory disabilities. It delivers 70% of aerosol particles within the one to four micron range....

Allied Aerosol Mask
$1.25
Clear soft vinyl construction and cotton coated strap provides patient comfort and accurate airway assessment and management....
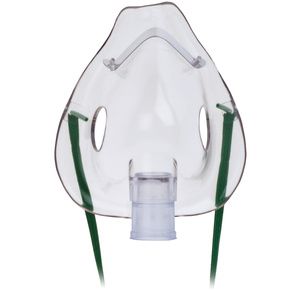
Hudson RCI Aerosol Masks
$2.37
Hudson RCI Aerosol Masks can be used with most handheld nebulizers for aerosol therapy. It has adjustable nose clip which assures comfortable fit....
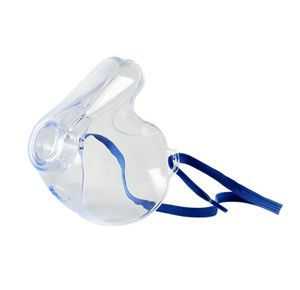
Pari Adult Aerosol Mask
$3.80
Pari Adult Aerosol Mask is designed with Pro-Vent technology, proven to deliver more medication while reducing facial and eye deposition. It has front-load design which directs aer...
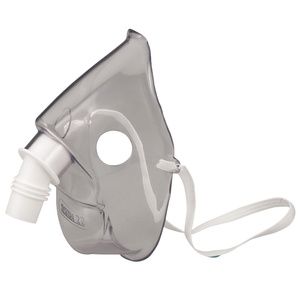
Respironics Sidestream Reusable Mask For Sidestream Nebulizer
$2.99
It is recommended for use with the Sidestream high-efficiency nebulizers....
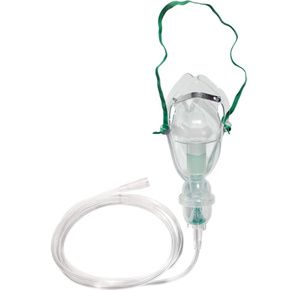
Sunset Healthcare Adult Aerosol Mask Kit
$3.99
It includes aerosol mask, 7ft supply tube and 6cc easy to assemble and disassemble nebulizer bottle....